Illuminating Cancer Cells During Surgery: A Worthy Olympus Investment
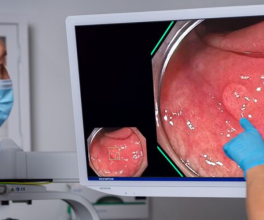
What if you could highlight tumors while the surgeon operates? That’s the idea behind CYTALUX® (pafolacianine), an imaging agent administered intravenously before surgery to help surgeons identify malignant and non-malignant lesions in adult patients with known or suspected lung cancer and ovarian cancer.1 On November 16, 2023, Olympus Innovation Ventures (OIV) announced its participation in the $30M Series C funding round to help West Lafayette, Ind. based On Target Laboratories (OTL). The team is working to accelerate commercialization of their novel compound—used in conjunction with near infrared surgical imaging devices.
CYTALUX is a molecular imaging agent with U.S. regulatory approval that illuminates lung and ovarian cancer as an adjunct during surgery, designed to improve surgeon’s ability to detect cancerous tissue.
Lighting up lesions
On Target Laboratories was founded in 2010, according to its website. Their "small molecular technology” is based on the pioneering work of Philip S. Low, PhD, Purdue University’s Presidential Scholar for Drug Discovery and the Ralph C. Corley Distinguished Professor of Chemistry. Their solution includes a near-infrared dye and targeting molecule that binds to receptors overexpressed on cancer cells with the goal of making cancer more visible in the OR.
CYTALUX received U.S. regulatory approval of its New Drug Application (NDA) in November 2021 for ovarian cancer, and a supplemental NDA for lung cancer in December 2022. In August 2023, On Target Laboratories was also granted a new technology add-on payment for CYTALUX.
“The ability of CYTALUX to illuminate lung and ovarian cancer during surgery is a stellar example of what we describe at Olympus as 'making the invisible visible.'”
Seeing the possibilities
“On Target Laboratories is at the forefront of a new wave of fluorescent agents that are game changers for surgical imaging,” said Nacho Abia, Chief Strategy Officer at Olympus. “The ability of CYTALUX to illuminate lung and ovarian cancer during surgery is a stellar example of what we describe at Olympus as ‘making the invisible visible,’” he said in an OTL press release.
The company plans to put the investment to good use in its efforts to commercialize the product. “This investment empowers us to accelerate the commercialization of our novel technology, further solidifying our position at the forefront of intraoperative molecular imaging,” said Ben Lundgren, President and CEO of On Target Laboratories. “This financing is another vote of confidence in our mission to illuminate cancer intraoperatively so it can be removed completely,” he said in an OTL press release.
Why we venture
OIV invests in passionate founders looking to revolutionize medical solutions. OIV’s focus is on customer-driven solutions in medical devices, diagnostics and digital health that complement Olympus’ market leading endoscopy devices. Reach out to ventures@olympus.com to learn more or let us know about the customer-driven medical solution you are building.
Important Safety Information
Infusion-Related Reactions
Adverse reactions including nausea, vomiting, abdominal pain, flushing, allergic reaction, elevation in blood pressure, indigestion, and chest discomfort were reported during the administration of CYTALUX. Your doctor may treat you with antihistamines and/or anti-nausea medication.
Risk of Misinterpretation
Errors may occur with the use of CYTALUX. Sometimes cells may light up even if they are not cancerous or those that are cancerous may not light up. Also, non-cancerous cells from other areas may light up, such as areas of the bowel, kidneys, lymph nodes, lungs, and inflamed tissue.
Pregnancy
CYTALUX may cause fetal harm when administered to a pregnant woman. There are no available human data to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Contact your healthcare provider with a known or suspected pregnancy.
Folate Supplementation Usage
Folic acid may reduce the detection of cancerous tissue with CYTALUX. Patients should stop taking folate, folic acid, or folate-containing supplements 48 hours before administration of CYTALUX.
Adverse Reactions
The most common side effects of CYTALUX reported in clinical trials were nausea (13%), vomiting (5%), abdominal pain (2%), flushing (2%), other infusion-related reactions (2%), allergic reaction (2%), elevation in blood pressure (1%), indigestion (1%), and chest discomfort (1%) during administration.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CYTALUX. For more information, ask your healthcare provider.
Call your doctor for medical advice about side effects. You may report side effects to On Target Laboratories at 1-844-434-9333 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See full Prescribing Information for more details.
Reference
1. CYTALUX [package insert.] Grand Rapids, MI: Grand River Aseptic Manufacturing; 2022.