A Patient-first Approach to Improve Bladder Control: Olympus Innovation Ventures Invests in Neuspera
Did you know that 1 in 6 adults suffer from over active bladder (OAB) with symptoms such as urinary incontinence, urgency and nocturia?1 That is why Steffen Hovard, Alexander Yeh and the team behind the Neuspera Medical, Inc. designed their Implantable Sacral Neuromodulation (SNM) System as a minimally invasive implant and convenient external wearable device that aims to provide relief from the symptoms of OAB that may impact a patient’s quality of life.
Olympus Innovation Ventures (OIV) recently announced a Series D investment in Neuspera Medical, Inc. This move underscores OIV’s commitment to investing in pioneering minimally invasive solutions that have the potential to enhance patient care and quality of life.
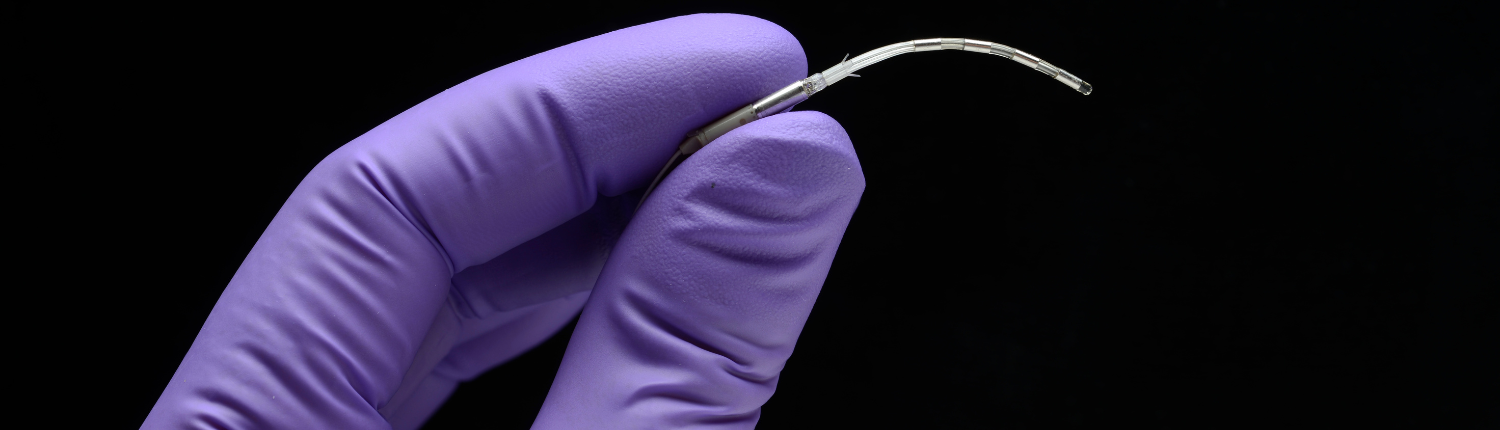
Neuspera's Wireless, Wearable SNM System
Neuspera looks to empower patients in the treatment of urinary urge incontinence (UUI) – a symptom of OAB – with its innovative implantable sacral neuromodulation (SNM) system.
At the heart of Neuspera's technology is an ultra-miniaturized implant and patented Mid-Field Powering capability that enables it to be powered wirelessly via an external wearable device. The system's discreet design and minimally invasive nature is meant to offer a patient-centric approach to managing UUI.
“As a partner of choice for innovators in urology, we are thrilled to invest in the Neuspera team as they work to bring the Neuspera System through FDA approval and to the market,” said Gabriela Kaynor, President of Olympus Innovation Ventures and Chief Strategy Officer at Olympus Corporation. “Based on critical unmet market need and patient demand, changes in society guidelines, and positive feedback from patients and physicians in clinical trials, we are excited to invest in the team as they build their innovative Neuspera System for OAB.”
Funding the Goal of Regulatory Approval, Next Steps
The Series D funding is intended to support Neuspera through the FDA premarket approval process. The goal is to reach this milestone in efforts to provide widespread access to the SNM system, designed to help patients find relief and gain confidence with a discreet system to manage symptoms of UUI.
“We’re excited to bring new partners on board as we approach the next significant milestone of submitting the Neuspera System for regulatory approval,” said Steffen Hovard, CEO of Neuspera Medical. “The confidence and conviction of our new and existing investors, like Olympus Innovation Ventures, demonstrates the strength and potential of our platform technology.”
Olympus Innovation Ventures' investment in Neuspera is a testament to the company's potential to transform the lives of patients suffering from UUI. As Neuspera works toward regulatory approval and commercialization, Olympus is proud to be a part of this journey towards a brighter future for those affected by this debilitating condition.
1. Stewart et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003 May;20(6):327-36.