Seven Reasons Why the HDTV in Your Living Room Should Not Be Used in a Procedure Room
Ever hear the one about the patient who tried to connect his smartphone to the monitor in the procedure room? What comes next isn’t a punchline but more of a caveat: “Most consumer-grade monitors sold today have some kind of network functionality, which presents a major risk to patient safety, as well as a security risk if protected health information (PHI) is accessible outside of hospital IT firewalls on connected devices,” according to Blake Pena, Product Manager for Image Chain and Accessories at Olympus. In 2024, Consumer Reports began rating consumer-grade TVs based on data privacy and security, in addition to overall picture quality (see “Best TVs of 2024”).1
The following are common specifications for a medical-grade monitor:
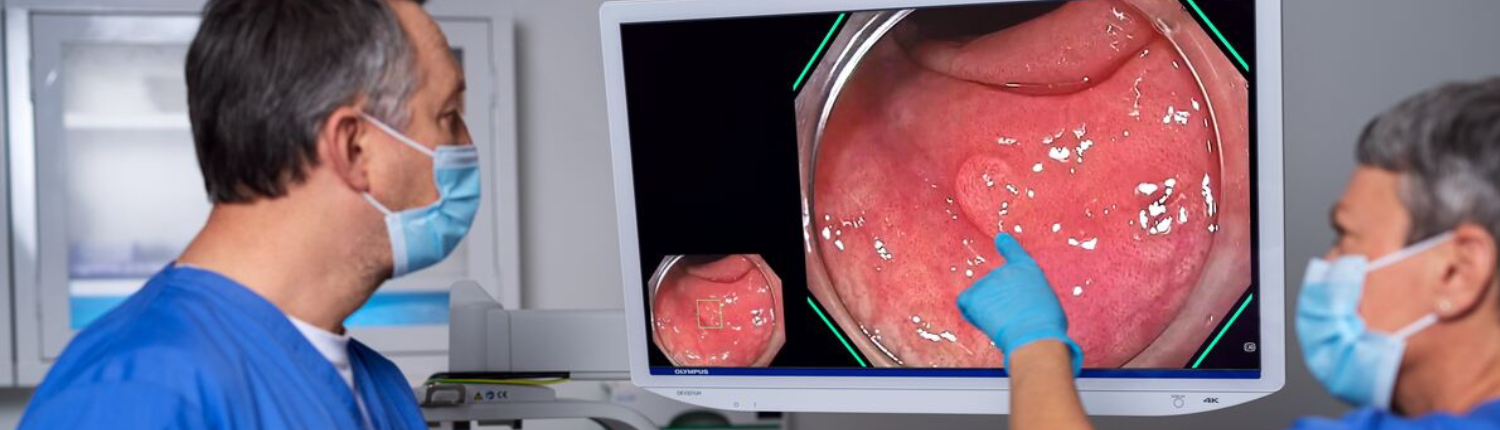
- Regulatory clearance as a medical device—Medical devices connected to a display monitor often require use of a medical-grade monitor, such as the OEV321UH 4K ultra high-definition LCD monitor, which is used with the Olympus® EVIS X1TM CV-1500 video system center during endoscopic procedures.2-4
- User friendly design—Display monitors should not introduce complexity to the procedure room. For example, the OEV321UH monitor includes a full flat display and detachable cable cover for easy cleaning, and the access window on the cable cover makes cabling easy.3
- Inputs/Outputs—“Simply put, you’re not likely to find 12G/3G input/output on a consumer device,” explained Pena. “You will definitely find an HDMI output, but you don’t have the same capabilities for cloning out or PIP/POP [picture-in-picture/picture-out-picture video] that a procedure room requires.”3
- Optimized for infection control—“Materials in procedure rooms should be validated with hospital disinfection products,” Pena cautioned. These same disinfection products could degrade consumer-grade equipment. Recommendations for cleaning consumer-grade LCD monitors are limited to a microfiber cloth and distilled water, with one manufacturer recommending only a 100:1 ratio of water to soap.5
- Natural color and optimized contrast—"Medical-grade monitors are designed with configurations complementary to their device counterparts,” said Pena. For example, he noted the OEV321UH 4K ultra high-definition LCD monitor features custom configurations for contrast, color, and brightness, as well as special features such as Advanced Image Multiple Enhancer (A.I.M.E.TM) technology, all of which are designed for high-quality imaging reproduction when used with the EVIS X1 endoscopy system.3,4
- Longevity—“Medical monitors use LCD technology as opposed to current trends in consumer products using LED/OLED,” according to Pena. “LCD technology is the least prone to ‘burn-in’ when a static image is left on the screen,” he said.6,7 “Think of the text that displays in the corner while a physician is scoping.”
- Electrical Standards—Medical monitors are tested to meet the International Electrotechnical Commission (IEC) medical electrical standard IEC 60601-1, in which AC and DC power is achieved using the appropriate conversion cable based on the standard, according to Pena. Standards such as IEC 60601-1 certifications also cover patient safety during medical equipment use.8,9
When it comes to watching the big game or this year’s blockbuster special-effects movie, there’s a lot of impressive technology available. But for endoscopic procedures that may benefit from specialized, proprietary imaging, physicians should stick with equipment that’s made for the job and configured for use within a facility or treatment center’s IT security firewall.
References
1. ConsumerReports.org. Best TVs of 2024. Updated February 9, 2024. Accessed April 23, 2024. https://www.consumerreports.org/electronics-computers/tvs/best-tvs-of-the-year-a3862868628/
2. FDA.gov. Computerized Labor Monitoring Systems – Class II Special Controls Guidance for Industry and FDA Staff. Issued April 24, 2007. Accessed April 11, 2024. https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/computerized-labor-monitoring-systems-class-ii-special-controls-guidance-industry-and-fda-staff#5
3. Data on file with Olympus (DC00438057).
4. Data on file with Olympus as of 4/2023.
5. ConsumerReports.org. How to Clean Your Flat-Screen TV. Updated March 26, 2024. Accessed April 23, 2024. https://www.consumerreports.org/electronics-computers/tvs/how-to-clean-your-flat-screen-tv-a1684280248/?itm_source=parsely-api
6. Gordon, W. Is 'Burn-In' Still an Issue on TVs and Monitors? Lifehacker. Posted February 6, 2013, Accessed April 24, 2024.
7. Archer, J. The OLED Screen Burn Debate – Everything You Need To Know. Forbes. Posted December 4, 2018. Accessed April 24, 2024.
8. ISO.org. IEC 60601-1-11:2015 Medical electrical equipment. Reviewed in 2020. Accessed April 23, 2024. https://www.iso.org/standard/65529.html.
9. Intertek.com. IEC 60601: Product Safety Standards for Medical Devices. Accessed April 24, 2024. https://www.intertek.com/medical/regulatory-requirements/iec-60601-1/